Monitoring Your Pulse While You SleepA Clinical Study Using Apple Watch
Keio University Hospital and the Keio University School of Medicine have conducted a clinical study using the Apple Watch, the “Apple Watch Heart Study“.
We are currently analyzing the data collected.
Thank you very much for your cooperation.

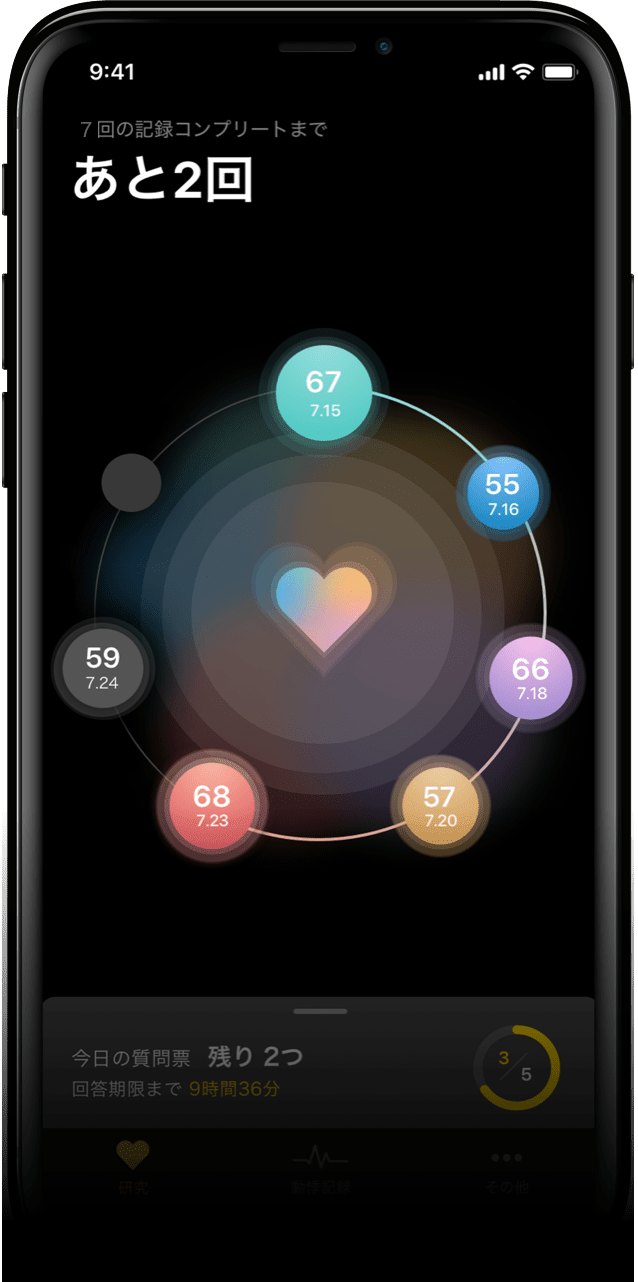
Seven-day clinical study to record pulse
and palpitations while sleeping
The purpose of conducting this clinical study was to
investigate the subjects’ pulse rate while resting and
sleeping; the study also involved asking the
participants
answer questionnaires, mainly to evaluate the
relationship
between sleep, alcohol consumption, and stress,
and to
clarify when an ECG should be recorded.
-
Wear Apple Watch
while sleeping -

Answer
questionnaires -

Record ECGs
and palpitations
Objectives of the “Apple Watch Heart Study”
Now that an ECG can be recorded on the Apple Watch in Japan, it is expected that self-management of health conditions will improve. However, an ECG does not involve data that can be safely recorded only once or at random times. If an ECG is recorded when one is experiencing a heart problem, it is possible determine whether the patient’s condition is normal. If an ECG is recorded when the patient is experiencing symptoms, it is possible to determine whether they are heart-related. However, if there are no symptoms, it is difficult to determine when to record an ECG. Therefore, this clinical study was conducted with the aim of analyzing the influence of lifestyle factors, such as sleep, alcohol consumption, and stress, on heart health using various types of healthcare data that can be measured by the Apple Watch and the subjects’ responses to the questionnaire. We also aimed to clarify the answer to the question: “When should I record an ECG?”
Why do we need to monitor our health in our sleep?
The heart continues to beat at night. While you are asleep, you will not notice any abnormalities in your heartbeat. Therefore, in this clinical study, you will be asked to wear an Apple Watch while sleeping. The goal of this research is to monitor your pulse rate while sleeping. Prior to conducting this clinical study, Keio University Hospital and the Keio University School of Medicine initiated a clinical study using the “Apple Watch Heart Study: Keio Edition.” This study will be conducted on atrial fibrillation patients receiving treatment at Keio University Hospital. By conducting an analysis of the data recorded by the Apple Watch, we aim to develop an algorithm to detect the optimal timing for recording a subject’s pulse and ECG when attempting to detect abnormalities. The goal of this clinical research is to evaluate the algorithm to be constructed in Keio University’s version of the study and to build a system that can accurately notify us of the timing of heart abnormalities. We ask for your cooperation so that we can properly utilize home healthcare data to enhance professional medical care.
Details of your role in the research
Please review the study contents in the research application and indicate whether you agree to participate in the study. Please wear an Apple Watch while you sleep and complete the questionnaire upon waking. Please record any symptoms related to palpitations, such as a pounding feeling, jumping heartbeat, rapid pulse, or any other heart-related discomfort. The study’s participation period is seven days.
How to spend your time during the participation period
-

Before bedtime
Wear your Apple Watch to bed.
The Heart Rate Center is used to measure your heart rate while you sleep. -
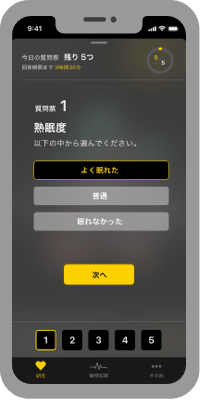
When you get up
When you wake up, please answer five simple questions every day.
We will send you a brief comment based on your measurements. -
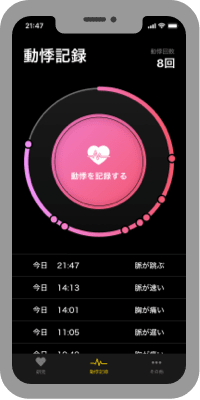
During the day
Record your heart palpitations in the app when you feel them.
-
For 7 days
Please carry out 1-3 for 7 days.
If you continue to take daily measurements, you will be able to look back from the app.
Participation in the study
Registration period for participants has been closed.
The application will remain available, but no information will be sent to us.
Thank you very much for your cooperation.
The results of the study will be published as soon as possible after the academic congress report.
Patients who meet the following criteria were asked to participate in the study.
- You must be at least 20 years old and use an iPhone or Apple Watch
- You must be able to download the research application from the App Store (Japan).
- You must be a Japanese citizen.
- You must be able to understand Japanese.
- You must consent to wear an Apple Watch for seven days while sleeping and be able to answer the questionnaire.
Download
Terms of Use:
The information stored or displayed in the research application should not be construed as medical advice or be used as a substitute for professional medical judgment. Always consult your healthcare provider for guidance regarding health-related decisions.
FAQ
- QIs there any cost to participate in the study?
- AThere is no cost to participate in the study.
- QDo I need to own an Apple Watch to participate?
- AYou must have access to an Apple Watch (Series 4 or later) with watchOS version 7 or later.
- QDo I need to own an iPhone to participate?
- AYou must have access to an iPhone (8 or later) with iOS version 14.0 or later.
- QCan I stop participating midway through the study?
- AEven if you have agreed to participate in this study, you may withdraw your consent and discontinue participation at any time. If you withdraw your consent, the collection of data from the research application will cease. We will also destroy any data from you that is already stored on our servers.
- QHow will my personal information be managed?
- AIt is impossible to identify individuals based on the data collected in this research, but we will handle the issue of privacy carefully and take precautions to prevent any personal information from being leaked to third parties. Everyone involved in the research will handle the information obtained in compliance with the provisions of the Act on the Protection of Personal Information.
- QHow will the data be presented?
- AWe plan to present the data at conferences and in papers. When the compiled information is published in medical journals, databases (UMIN-CTR), etc., it will be processed using statistic software and no personal information will be disclosed.
Research organization
In 2018, Keio University Hospital was selected for participation in the “Advanced Diagnosis and Treatment System by AI (Artificial Intelligence) Hospital” project, which is part of the Cabinet Office’s Strategic Innovation and Creation Program (SIP) to realize Society 5.0 and involves promoting IT and AI use in hospitals. This clinical research is supported by this AI hospital project.
- Principal Investigator: Professor Masahiro Jinzaki M.D.
Department of Radiology (Diagnostics), Keio University School of Medicine - Chief Investigator: Takehiro Kimura M.D.
Department of Cardiology, Keio University School of Medicine

Support
This clinical research has received the support of Apple in the development of the research application.
-

Biosense Webster Inc.
-

atsurae, Inc.
-

JMDC, Inc.
